History of InCROM Group
InCROM Group consisting of CRO, SMO and site network services started our full-scale clinical trial support services in 1983. Founded in 1975 as RABITON Farm and later incorporated as RABITON Institute, Inc., we evolved from rabbit farming to non-clinical research supporting the conduct of non-clinical studies such as reproductive toxicity studies, and ocular mucosa and skin irritation studies in rabbits. In 1983, we also supported the establishment of Osaka Pharmacology Research Clinic, a facility specialized in clinical trials. As part of the InCROM Group affiliated clinical trial site network, together with Heishinkai Medical Corporation and other core affiliates, and InCROM SMO, InCROM CRO (incorporated in 2010) provides clinical trial support services across a wide array of indications.
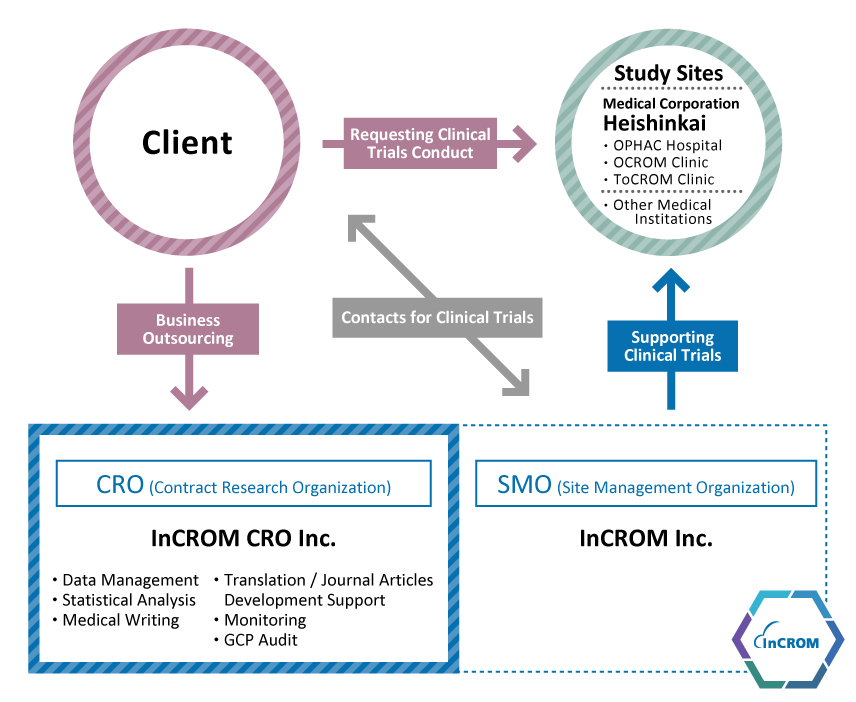
InCROM CRO
Being part of the InCROM Group, InCROM CRO leverages on the group resources and collaborations with InCROM (SMO) and affiliate Heishinkai Medical Corporation (Heishinkai) to offer our clients synergies leading to clinical conduct efficiency enhancement. Both InCROM (SMO) and Heishinkai have a wealth of experience and knowledge in clinical pharmacology, allowing us to provide high quality CRO services on tasks such as clinical trial planning consultation, protocol development, clinical trial schedule management, monitoring, data management, statistical analysis, CSR, and GCP audits.
An equally valuable differentiator is our being a smaller organization managed by a strong team of industry and InCROM experienced experts, which enables us to offer clients with a high level of flexibility and responsiveness to any urgent and/or irregular scheduling or other needs. We also contract with partners to offer clinical trial services and solutions, as well as offer on-demand and/or functional service solutions.
Key Strengths
• Extensive experience in clinical pharmacology
• High level of flexibility and responsiveness
• Service speed and quality
InCROM Group Experience
Below is a list of InCROM supported clinical trials (majority conducted at Heishinkai Medical Corporation sites)
(As of June 1, 2023)
| Month/Year | Study Type | Site (Heishinkai site*) | CRO Work Scope* |
| May 2023 | Specified Clinical Research | OPHAC Hospital* | MO, MW, AU |
| Apr 2023 | Phase Ⅰ | OPHAC Hospital* | MO, DM, ST, MW, AU |
| Mar 2023 | Phase Ⅲ | Other medical institutions | MO, DM, ST, AU |
| Nov 2022 | Phase Ⅲ | OPHAC Hospital* OCROM Clinic* Other medical institutions |
MO, DM, ST, MW |
| Nov 2022 | Clinical research | OPHAC Hospital* | MO, DM, MW |
| Jul 2022 | Phase Ⅲ | OCROM Clinic* ToCROM Clinic* |
MO |
| Jun 2021 | Phase I | OPHAC Hospital* | MO, DM, ST, MW, Audit |
| Apr 2021 | Phase I / Ⅱ | OPHAC Hospital* OCROM Clinic* |
MO,audit |
| Apr 2021 | BE | OPHAC Hospital* | MO |
| Feb 2021 | Phase I | OPHAC Hospital* | MO |
| Nov 2020 | Phase Ⅱ | OCROM Clinic* | MO, Audit |
| Nov 2019 | Phase I | OPHAC Hospital* | MO, Audit |
| Oct 2019 | Phase I | OPHAC Hospital* | MO, DM, ST, MW, Audit |
| Oct 2019 | Phase I | OPHAC Hospital* | MO, DM, ST, MW, Audit |
| Sep 2019 | Phase I | OPHAC Hospital* | MO, DM, ST, MW |
| Jun 2019 | Phase Ⅲ | Other medical institutions | MO, DM, ST, MW, Audit |
| Jun 2019 | BE | OPHAC Hospital* | MO |
| Apr 2019 | Phase I | OPHAC Hospital* | MO, DM, ST, MW, Audit |
| Jan 2019 | Phase I | OPHAC Hospital* | MO |
| Dec 2018 | Phase I | OPHAC Hospital* | MO, DM, ST, MW |
| Oct 2018 | BE | OPHAC Hospital* | MO |
| Oct 2018 | Phase I | OPHAC Hospital* | MO, DM, ST, MW, Audit |
| Aug 2018 | BE | OPHAC Hospital* | MO |
| Jul 2018 | BE | OPHAC Hospital* | MO, DM, ST, MW |
| Mar 2018 | Phase Ⅲ | OPHAC Hospital* OCROM Clinic* ToCROM Clinic* Other medical institutions |
MO, DM, ST, MW, Audit |
| Jan 2018 | Phase I | OPHAC Hospital* | MO |
| Oct 2017 | Clinical research | OPHAC Hospital* | MO, DM, ST, MW |
| Sep 2017 | Clinical research | OPHAC Hospital* ToCROM Clinic* |
MO, DM, ST, MW, Audit |
| Aug 2017 | Clinical research | OPHAC Hospital* | MO, DM, ST, MW |
| Aug 2017 | Phase Ⅱ | OPHAC Hospital* ToCROM Clinic* |
MO, DM, ST, MW, Audit |
| Jul 2017 | Clinical research | OPHAC Hospital* | MO, DM, ST, MW |
| Jul 2017 | BE | OPHAC Hospital* | MO, DM, ST, MW, Audit |
| Apr 2017 | Phase Ⅱ | OPHAC Hospital* Other medical institutions |
MO |
| Mar 2017 | Clinical research | Other medical institutions | MO, DM, MW |
| Jan 2017 | BE | OPHAC Hospital* | MO, DM, ST, MW |
| Dec 2016 | Clinical research | Other medical institutions | MO, MW |
| Dec 2016 | Clinical research | OPHAC Hospital* | MO, DM, ST, MW |
| Nov 2016 | Phase Ⅲ | OPHAC Hospital* ToCROM Clinic* |
MO, DM, ST, MW |
| Sep 2016 | BE | OPHAC Hospital* | MO, DM, MW |
| Sep 2016 | BE | OPHAC Hospital* | MO, DM, ST, MW |
| Aug 2016 | Clinical Research | OPHAC Hospital* | MO, DM, ST, MW |
| Aug 2016 | Clinical research | OPHAC Hospital* | MO, DM, ST, MW |
| Aug 2016 | Clinical research | Other medical institutions | MO |
| Feb 2016 | Phase I | OPHAC Hospital* ToCROM Clinic* |
MO, DM, ST, MW |
*MO: monitoring, DM: data management, ST: statistical analysis, MW: medical writing

